Abstract
Rationale Long-term clinical remissions of hematologic malignancies after allogeneic hematopoietic stem cell transplantation (HSCT) largely rely on the graft-versus-leukemia effect (GVL). In a fully HLA-matched setting, the GVL effect is mediated primarily by T cell immune responses against host minor histocompatibility antigens (MiHAs) found on the surface of malignant cells. Upon relapse of leukemia following HSCT, donor lymphocyte infusions (DLI) have unfortunately failed to exert efficient GVL activity. This is likely due to the insufficient number of anti-MiHA T cells within DLIs and to the risk of graft-vs-host disease (GVHD) that prevents the administration of high numbers of T cells. To generate potent and selective anti-leukemia activity, we have developed a strategy for the ex vivo selection and expansion of T cells targeting MiHAs preferentially expressed on hematopoietic cells (Perreault, Leukemia 2016). Thus, these cells should display augmented GVL immune reactivity while harboring a low risk of causing GVHD. To our knowledge, this is the first clinical study targeting several different molecularly defined MiHAs and on different HLA molecules.
Patients and Methods In an open-label, multi-center phase 1 clinical trial (CR-MIHA-001; NCT 03091933), patients with hematologic malignancies who relapsed after an allogeneic HLA-matched HSCT were treated with sibling donor-derived, ex vivo- expanded T cells selected and expanded for reactivity toward tissue-restricted recipient MiHAs (GLIDE: Guided Lymphocyte Immunopeptide Derived Expansion). After donor cell collection, patients received fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 for 3 days followed by infusion of GLIDE, consisting of donor T-lymphocytes expanded ex vivo following exposure to dendritic cells coated with immunogenic peptides (MiHAs) present on the surface of host hematopoietic cells, at a single target dose of 4x107 viable T-cells/m2. A second dose of GLIDE, aiming at 3-5 times the original dose, could be administered to patients at a minimum of 6 weeks after the first infusion. GLIDE cell characteristics were evaluated using multi-parameter flow cytometry and TCR sequencing. Patients were followed up for one year post-GLIDE infusion for toxicity and clinical outcome measures.
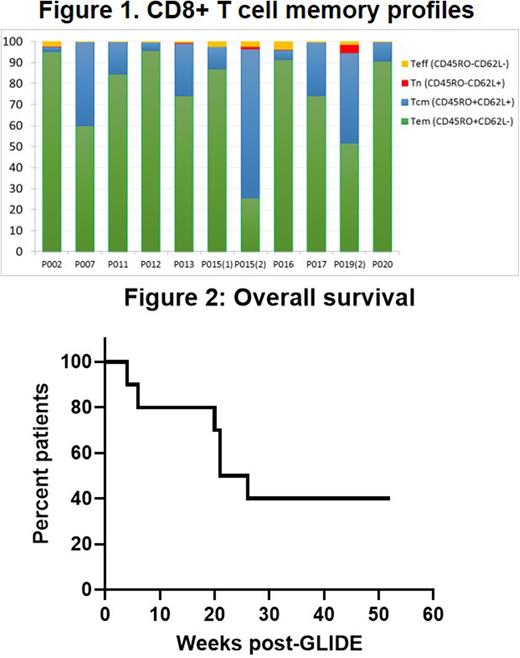
Results Ten patients, median age of 58 (range 33-69), 4 females/6 males with AML (n=7), NHL (1), CLL (1), and myelofibrosis (1) were treated with GLIDE. These patients had relapsed at a median of 12 months (range 5-126 mo) after allogeneic HSCT. GLIDE cells were personalized to target single MiHAs in 9 patients and two MiHAs in 1 patient. Overall, GLIDE cell products were directed at 9 different MiHAs on 6 different HLA-class 1 molecules (HLA-A02:01, A24:02, B07:02, B18:01, B44:02, and B44:03). A first dose of GLIDE was administered at 4.0x107 CD3+ cells/m2 to 8 patients, and 2 patients received slightly lower doses (2.6 and 3.7 x 107 CD3+ cells/m2). Final GLIDE products consisted mainly of T-cells (median: 98,7%), with CD8>CD4>NKT>NK cells. Most CD8+ T cells were effector memory and central memory T cells (Figure 1). At the end of the expansion period, CD8+ T cells expressed activation markers (CD25+ = 67%) with low levels of PD1+, PDL1+, CD57+ and KLRG1+ as markers of exhaustion. Not surprisingly, GLIDE cells had a more restricted TCR β chain profile than initial lymphopheresis cells. Six patients received a second dose of GLIDE (mean: 12 x 107 CD3+ cells/m2) at 53 days after the first infusion (median, range: 48-118 days). There were no infusional toxicities, and cells were well-tolerated by patients. Only one patient developed thrombocytopenia and subsequent chronic GVHD. There were no other adverse events potentially related to GLIDE. According to TCR β sequencing data, T cells present in the adoptively transferred anti-MiHA GLIDE product persisted in peripheral blood up to one year after infusion. Survival at one year was observed in 4 patients with 3 patients in sustained remission (Figure 2).
Conclusion This pilot study demonstrates the feasibility of anti-MiHA T cell ex vivo generation (GLIDE) and safety of its administration to patients relapsing after allogeneic HSCT, as well as the clinical potential of these cells. The persistent remissions and low occurrence of GVHD support further development of GLIDE to target acute myeloid leukemia and other hematologic malignancies.
Disclosures
Roy:Kiadis Pharma: Consultancy, Honoraria; Knight Therapeutics: Consultancy; Pfizer: Speakers Bureau. Leber:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AMGEN: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Couture:Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Bambace:AbbVie: Consultancy; Bristol Myers Squibb: Consultancy. Cohen:ExCellThera: Consultancy, Patents & Royalties, Research Funding. Roy:ExCellThera: Patents & Royalties. Sauvageau:ExCellThera: Consultancy, Current Employment, Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; BMS: Research Funding. Veilleux:Kite-Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Busque:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal